Dyne Therapeutics, Inc. (DYN) is a clinical-stage biotechnology company focused on developing transformative therapeutics for people living with serious muscle diseases caused by genetic mutations. The company is headquartered in Waltham, Massachusetts, and leverages its proprietary FORCE™ platform to create innovative oligonucleotide therapeutics designed to deliver drugs directly to muscle tissue, thereby overcoming significant challenges in treatment efficacy.
Dyne’s pipeline includes several promising programs targeting muscle diseases such as myotonic dystrophy type 1 (DM1), Duchenne muscular dystrophy (DMD), and facioscapulohumeral muscular dystrophy (FSHD). The company is actively conducting clinical trials for DYNE-101 and DYNE-251, which are designed to address DM1 and DMD, respectively. These trials, named ACHIEVE and DELIVER, have shown positive initial data, indicating improvements in key disease biomarkers and functional endpoints, which are crucial for disease management and patient quality of life.
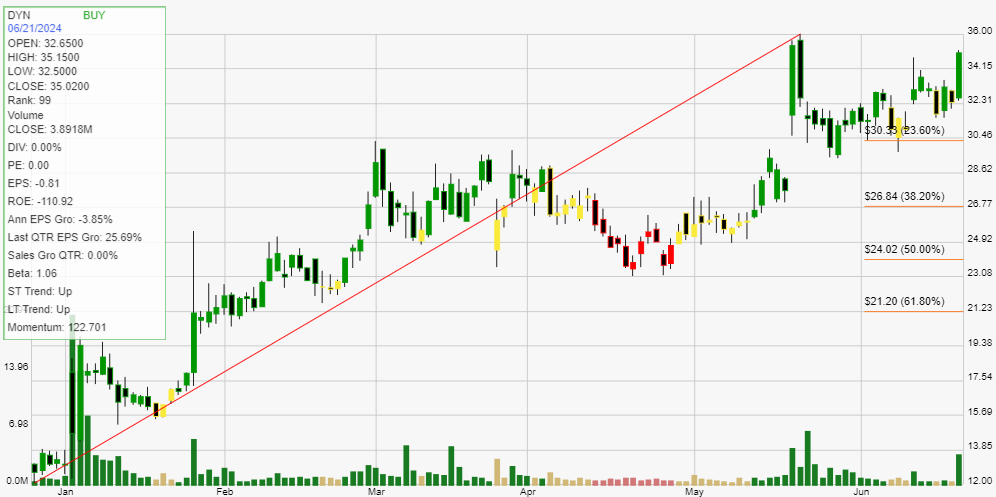
The key drivers of growth for Dyne Therapeutics are its advanced clinical trials, strategic leadership, and robust financial health. With substantial funding secured through public offerings, the company is well-positioned to continue its research and development activities. The anticipated data from higher dose cohorts in the ACHIEVE and DELIVER trials, expected in the second half of 2024, represent significant milestones that could pave the way for regulatory approvals and commercial launch. Moreover, the appointment of experienced leaders in the biotechnology field further strengthens the company’s strategic direction and operational execution